MAFLD & MASH
An out-of-the-box vision to treat metabolic-associated fatty liver disease (MAFLD)
Overview
Metabolic associated fatty liver disease (MAFLD) is closely linked to dietary caloric excess and a sedentary lifestyle. MAFLD is defined as hepatic steatosis (fat storage) associated with overweight or obesity, diabetes mellitus (T2DM), metabolic syndrome (high waist circumference, hypertension, hypertriglyceridemia, and low- high-density cholesterol). Cardiovascular disorders are the leading cause of mortality in patients with MAFLD, followed by extrahepatic malignancies. MAFLD is also associated with chronic kidney disease (CKD), polycystic ovarian syndrome (PCOS), obstructive sleep apnea (OSA), extrahepatic malignancies, osteoporosis, and cognitive disorders.[1]
MAFLD has become the leading cause of chronic liver disease representing a significant burden on the worldwide healthcare system. Its prevalence is estimated of 25% overall and of nearly 60% in T2D patients[2] , with an increased risk of developing hepatocellular carcinoma. It is estimated that the number of people suffering from MAFLD will increase by 63% by 2030[3] .
MASH (metabolic dysfunction–associated steatohepatitis) represents the aggressive form of MAFLD. They have a higher rate of fibrosis, progress more rapidly to cirrhosis and to more liver complications with a higher mortality rate.
[1] E. Kaya & Y. Yilmaz. J. Clin. Transl. Hep. 2022 doi: 10.14218/JCTH.2021.00178
[2] Younossi ZM et al. J Hepatol. 2019. doi: 10.1016/j.jhep.2019.06.021.
[3] Estes C. et al. 2018. doi: 10.1002/hep.29466.
MAFLD and Adiponectin
Hypoadiponectinemia correlates with MAFLD
A statistical study revealed that patients with MAFLD have lower serum adiponectin levels than the control group[1].
High Molecular Weight adiponectin correlates inversely with markers of liver injury[2].
[1] Sargin H. et al. World J Gastroenterol. 2005. doi: 10.3748/wjg.v11.i37.5874.
[2] Liu Y. et al. J Clin Endocrinol Metab. 2007. doi: 10.1210/jc.2007-0890.
Mode of Action of Adiponectin hepatoprotection
ANTI-INFLAMMATORY PROPERTIES
Inflammatory cytokines are key mediators of hepatic inflammation, cell death, and fibrosis.
Evidence reveals that adiponectin levels are inversely related to pro-inflammatory cytokines, such as IL-6, which are produced by Kupffer cells and hepatic stellate cells (HSCs). Conversely, adiponectin levels are directly related to anti-inflammatory cytokines, such as IL-10.
Adiponectin improves NASH and liver fibrosis by suppressing the activation of Kupffer cells and HSC[3].
[3] Adachi M & Brenner DA. Hepatology. 2008. doi: 10.1002/hep.21991.
ANTI-OXYDATIVE STRESS
Patients with NASH have an accumulation of reactive oxygen species (ROS), which oxidize fat deposits and form lipid peroxidation products. These products cause steatohepatitis, necrosis, inflammation, and fibrosis. Adiponectin decreases levels of these products by regulating hepatic mitochondrial function[4].
[4] Buechler C et al. World J Gastroenterol. 2011. doi: 10.3748/wjg.v17.i23.2801.
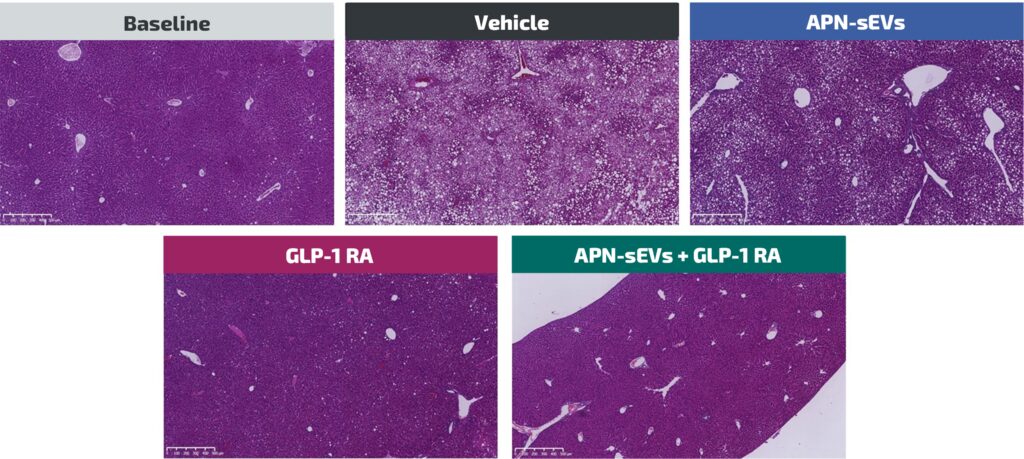
Indications of hepatoprotective effects of APN-sEVs
The specific impacts of APN-sEV observed on different signaling pathways involved in lipogenesis, gluconeogenesis, fatty acid B-oxidation and inflammation are paralleled with the observations of :
- A reduction of monocyte chemoattractant protein-1 (MCP-1) mRNA level under APN-sEV treatment alone.
- A sharp drop in liver triglyceride and free fatty acid levels specific to only APN-sEVs/GLP-1RA co-treatment.
- A reduction of liver inflammation and oxidative stress in the liver when APN-sEVs are combined to GLP-1RA.
- A steatosis reduction with the combination of APN-sEVs and GLP-1RA.
All these effects indicate that APN-sEVs combined to GLP-1RA are a promising candidate treatment for MAFLD and potentially for MASH.